In recent years, regulatory agencies such as the Food and Drug Administration (FDA) have expanded the level of testing required for certain products, such as medical devices and pharmaceuticals. Consequently, there is a consistent and growing demand for stability chambers to meet these stringent requirements.
A stability chamber is a specialized environmental chamber used in pharmaceuticals, food, cosmetics, and other industries, to simulate and assess the long-term effects of environmental conditions on products and materials.
Stability chambers are equipped with sophisticated control systems that maintain the desired temperature and humidity levels, simulating an environment that will reveal a product’s stability over an extended period. These environmental test chambers are essential to helping manufacturers and researchers understand how a product will perform under specific conditions to predict its quality, efficacy, and overall safety.
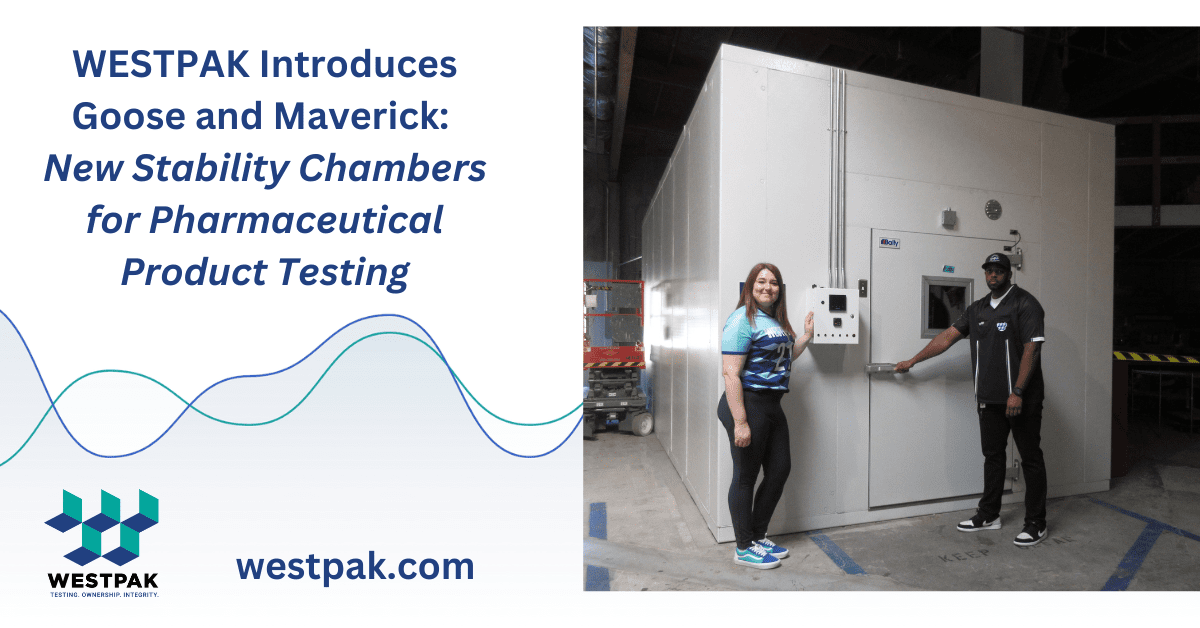
Recently WESTPAK introduced its newest addition to its accredited San Diego, California, testing laboratory: Goose. Goose, the company’s new walk-in size stability chamber, passed stringent QA checks, and was released to production for customer projects. The chamber has 700 ft3 of usable internal volume, a temperature range of -20OC to +70OC, and a humidity range of 20% to 75% RH.
Goose joins Maverick, WESTPAK’s other recently added stability chamber, released in 2022. Maverick boasts 600 ft3 of usable volume, and its temperature and humidity range matches Goose’s. These chambers are typically shared, enabling significantly reduced customer pricing. Maverick is currently set at +25OC and 60% RH.
These stability chambers are used for various purposes, including:
1. Shelf-life studies: Determining how long a product remains stable and safe under different environmental conditions before degradation starts.
2. Quality control: Ensuring products meet predefined quality standards throughout their lifespan.
3. Formulation development: Evaluating how changes in formulation or packaging affect product stability.
4. Regulatory compliance: Meeting industry-specific regulations and guidelines often requiring stability testing for certain products, such as pharmaceuticals.
5. Research and development: Studying the effects of temperature and humidity on new product formulations or materials.
Stability chambers are crucial in product development and quality assurance, helping manufacturers deliver safe, effective, and reliable products to consumers.
WESTPAK is committed to consistently providing unparalleled customer service and accelerating a manufacturer’s timeline to get products to market quickly. By increasing testing capacity, WESTPAK’s team of dedicated experts can ensure faster turn-around time for testing projects, resulting in a shorter time-to-market schedule.
Goose and Maverick join WESTPAK’s suite of 100+ environmental chambers. Read about ICH Stability Testing for Pharmaceutical Products here.
How can we help you with your test projects? Please get in touch with our Sales team to get a quote, information, or ask questions.